

In November, the Chinese startup Lonvi Biosciences announced the development of anti-aging pills that it said would allow people to live to 150. The remarks by company head Liu Qinghua came a few weeks after an infamous conversation in which the leaders of Russia, China, and North Korea were overheard talking about precisely that subject. “Forecasts show that there is a chance to live to 150 in this century,” Xi Jinping said at the end of a chat that, it turned out, was being recorded from the Chinese side. Over the past decade, scientists have indeed made significant progress in studying aging, yet none of the discoveries in the field have produced a reliable means of extending life. Despite the fast-growing market for supplements that supposedly promote longevity, clinical research still cannot confirm the effectiveness of new technologies, and the 120-year limit on maximum life span remains unbroken.
Content
How human life expectancy has risen
Chronological and biological age
Myths about extending life
How human life expectancy has risen
Until the 19th century, average human life expectancy rarely exceeded 32 years, and that held true almost everywhere throughout the history of civilization. This statistic reflected high child mortality rates, along with the prevalence of wars, famine, and pandemic. Of course, in Ancient Rome and elsewhere, there were plenty of people who attained a “death from old age” sometime in their 70s, but this was not the norm.
The situation began to change in the era of industrialization, when rapid advances occurred in the natural sciences. Urban populations grew, and public hygiene began to take shape. Quality of life improved as people gained access to more varied food, cleaner water, and better medical care.
A sharp rise in life expectancy came in the 20th century when humanity learned to control infections. The invention of antibiotics, mass vaccination, and advances in neonatal care transformed the statistics. By 2023, average life expectancy worldwide had reached 70, and in many countries it surpassed 80.
The age record, belonging to Frenchwoman Jeanne Calment (1885-1997), stands 122 years and 164 days. But can 120 years be called a limit? Will average life expectancy continue to rise? To answer these questions, it is first necessary to understand what aging is.
Aging
There is no single definition of “aging,” since it is a complex biological process in which the body gradually loses its ability to recover. Cells accumulate damage, systems stop working in sync, and at some point the body can no longer compensate for internal disruptions.
Aging is a complex process in which the body gradually loses its ability to recover
In 2013, Spanish researcher Carlos López-Otín and his colleagues proposed the concept of “hallmarks of aging” — key biological processes that shape the picture of the body’s deterioration. The academic community embraced this approach, and over the ensuing ten years more scientific papers were published on the topic than had been in the entire 20th century. In 2023, drawing on accumulated data, researchers revised their concept, dividing the hallmarks of aging into three levels: molecular, cellular and systemic.
At the molecular level, aging begins with DNA damage, disruptions in protein metabolism, and failures in the cellular waste-clearing system (autophagy). These are fundamental mechanisms that ensure stability and “clean-up” inside the cell. When they break down, the body loses its ability to repair itself.
At the cellular level, secondary changes occur: mitochondria, which supply cells with energy, begin to work inefficiently; cells reconfigure their metabolism and sometimes fall into a state known as senescence, in which they no longer divide but continue to emit inflammatory signals. These reactions initially protect the body, but with age they become destructive.
At the systemic level, the consequences of all these disruptions emerge: tissues lose their ability to renew, the body can no longer respond adequately to stress and disease, and age-related illnesses develop — pulmonary fibrosis, dementia, hypertension, and others.
When the body can no longer respond adequately to stress and disease, age-related illnesses develop
A failure in one system pulls others down with it, and intensifying efforts to fight such failures opens new risks. For example, the buildup of senescent cells is harmful because they release inflammatory substances that disrupt the function of neighboring cells. On the other hand, the aging process itself protects against cancer by preventing the division of cells with damaged DNA. The immune system is supposed to detect and remove such cells, but its ability to do so weakens with age because breakdowns accumulate there as well.
Chronological and biological age
When we say “he is 40” or “she is 60,” we mean chronological age — how many years have passed since a person was born. But this measure reflects the condition of the body only loosely. Some forty-year-olds might be winded after climbing a few flights of stairs, while others still run marathons at competitive times. That is why scientists increasingly speak of biological age — a more accurate characteristic that describes the body’s internal wear and tear.
Biological age can be seen as the body’s speedometer. It shows how fast we are moving along the path of aging. To assess it, researchers analyze many indicators: gene activity, metabolic byproducts, and the state of the microbiome among them. In recent years, thanks to big data, it has become possible to combine these indicators into unified models, making it possible to look at aging from a new angle.
Biological age can be seen as the body’s speedometer. It shows how fast we are moving along the path of aging
In August 2024, the journal Nature Aging published a study that caused a real stir in the field of gerontology. A team of scientists from Stanford University, led by Michael Snyder, analyzed thousands of blood and tissue samples to understand how molecular processes change with age. The result was unexpected. Aging had previously been viewed as a gradual and uniform decline of all bodily functions. But Snyder’s study showed that it is more of a stepwise process. There are two clear turning points at which the pace of aging accelerates sharply.
The first of these moments comes at about 44: levels of certain hormones fall, the body’s ability to repair tissue declines, and the metabolism becomes less efficient. The second jump occurs around age 60–65. By this point, the immune system has weakened, cells lose their ability to divide and regenerate, and accumulated damage crosses a critical threshold. As a result, characteristic signs of aging appear: chronic inflammation, increased fatigue, metabolic disruptions, and cognitive changes.
The body ages rapidly at 44 and again around 60–65
This view of aging changes the very approach to extending life. By tracking biological age and these acceleration points, it becomes possible to assess the body’s wear and determine when exactly intervention might prolong an individual person’s period of active health.
Myths about extending life
Gerontologists around the world are working to turn the concept of “hallmarks of aging” into concrete therapeutic strategies. But the press often exaggerates scientific progress. Every newly published study is inevitably accompanied by media headlines like “Russia promises to release anti-aging pills,” while the real results end up buried under misleading information. Some myths arise from excessive optimism, others from misinterpreting genuine discoveries.
Myth one: senolytics solve every problem
Scientists have long noticed that senescent cells — those that have stopped dividing but continue to exist — accumulate in the tissues of old animals. These cells, neither alive nor dead, release substances that interfere with the work of other cells. In the past, researchers could eliminate such cells only through genetic modification. In 2011, James Kirkland’s lab at the Mayo Clinic created the INK-ATTAC transgene, which triggered self-destruction of aging cells in laboratory mice. As a result, the mice lived longer and developed age-related diseases more slowly.
In 2015, the same team published a study in Aging Cell on a new class of drugs that were later called senolytics. Researchers identified a gene network in senescent cells that ensured their survival and selected compounds that selectively switched these networks off.
Just five days after receiving a single dose, the animals’ heart and vascular function improved. When the drugs were administered in courses to naturally or genetically accelerated aging mice, the animals not only lived longer but also stayed healthy: age-related diseases appeared later than before, bones fractured less often, and spinal cartilage was better preserved.
Just five days after a single dose of the drug, the animals’ heart and vascular function improved
Active research on senolytics led to the emergence of a new class of drugs — senomorphics. These compounds do not destroy aging cells but “calm” them by reducing the release of inflammatory signaling molecules through which such “zombie” cells harm their neighbors.
The first human trials of senolytic therapy began in 2019. A Mayo Clinic team administered the drug to nine patients with diabetic kidney disease. After 11 days, researchers recorded a decrease in the number of aging cells in fat and skin tissue and a reduction in blood levels of inflammatory markers. However, the authors stressed that it is still impossible to draw conclusions about the potential for rejuvenation or life extension.
Nevertheless, after publication, senolytics and senomorphics became a frequent topic in the press. As a result, since 2020 the market has seen the emergence of supplements and products containing combinations of substances that once showed some sort of anti-aging effect. Today there is a vast market offering a wide range of senolytic variations for any budget — one only needs to type the name of one of these compounds into a search bar.
Since 2020, the market has seen the emergence of supplements and products containing combinations of substances that at one time showed some anti-aging effects
Given that the dietary supplement market is less strictly regulated than the pharmaceutical market, the uncontrolled spread of senolytics worries doctors, as their long-term effects on the body remain insufficiently studied. Aging cells can also be beneficial. For example, they help with regeneration in cases of acute organ damage, so indiscriminate elimination could lead to undesirable consequences. Moreover, there are currently no good tools to assess the number of senescent cells in the human body in order to determine the right time to start gerontological therapy.
Finally, not all aging cells develop resistance to programmed “self-destruction” (apoptosis), meaning that senolytic drugs designed to target them are not universal and address only part of the problem. For instance, senolytics are ineffective against old mesenchymal stem cells, which require additional intervention in order to overcome their protection against programmed cell death.
Myth two: “tweak” the metabolism with pills and youth will return
Aging is accompanied by the accumulation of metabolic disruptions. Does this mean that if these disruptions are compensated for, youth will return? In short, no.
Here, as with research on senolytics, scientists first focused on drugs already used in medicine. The anti-aging effect is assessed in medications that regulate metabolism and are used to treat type 2 diabetes.
Once it became known that these drugs could promote weight loss, they gained extraordinary popularity. Prescriptions were issued not for their primary medical purpose, but for weight loss, which ultimately led to global supply shortages and price increases.
In the U.S. and the UK, regulators had to establish priority access to such drugs for those who needed them for medical reasons. In addition, numerous warnings were issued by the U.S. Food and Drug Administration (FDA) regarding the use of uncertified or counterfeit versions of these drugs, which pose health risks due to their unverified dosage, composition, and quality.
As soon as it became known that anti-diabetic drugs could promote weight loss, they gained extraordinary popularity
Gerontologists took notice of this class of drugs because practical use revealed that they positively affect age-related pathologies: cardiovascular disease, neurodegeneration, kidney disease, and cancer. However, all clinical trials have been conducted only among older adults with diabetes and obesity. Trials in healthy individuals have not been performed, meaning their impact on lifespan or biological age remains unproven.
Myth three: edit the genome and “reset” aging
The idea of editing the genome emerged in the late 1950s, when the structure of the DNA double helix was discovered. Over the next forty years, the first genetically modified animals, plants, and organisms were developed using radiation, chemical mutagens, and early recombinant DNA technologies. However, these approaches lacked precision.
Such attempts continued until 2012, when Jennifer Doudna and Emmanuelle Charpentier published a paper detailing the mechanism of the Cas9 enzyme in the bacterial CRISPR system. Acting like a molecular immune system in bacteria, Cas9 recognizes and destroys foreign genes. Doudna and Charpentier’s team proposed using this enzyme as molecular scissors to cut or replace genome fragments anywhere. In 2013, two independent research groups successfully edited the DNA of eukaryotic cells via this method.
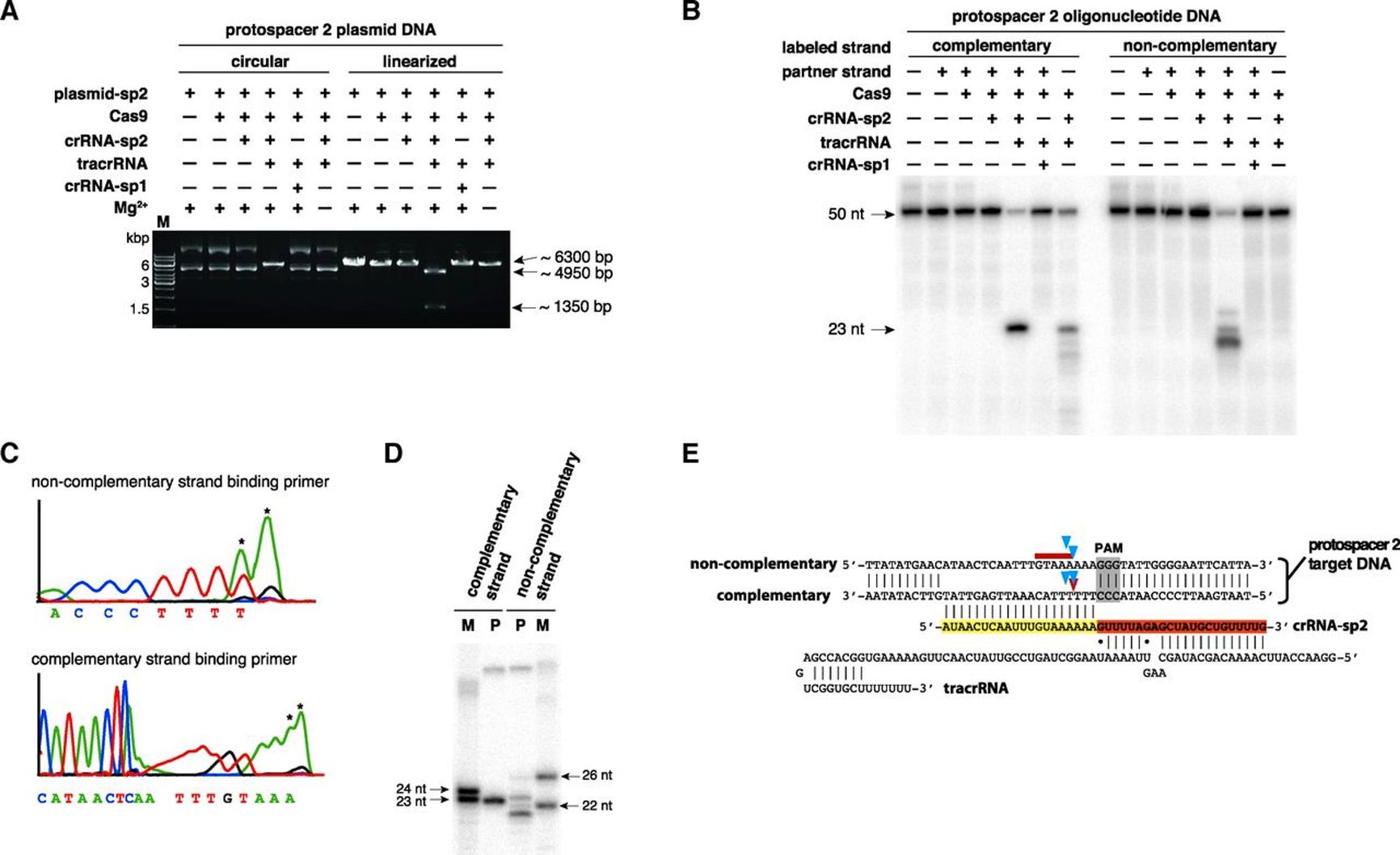
Infographic from the article by Jennifer Doudna and Emmanuelle Charpentier showing the mechanism of the Cas9 enzyme in the bacterial CRISPR system
To date, more than 70 clinical trials using CRISPR technology and related genome-editing methods have been registered. These trials cover a wide range of diseases — from hereditary and metabolic disorders to cancer and neurological conditions.
However, this technology also has serious limitations, as altering a single gene can trigger unpredictable reactions. Scientists still have limited ability to track unintended changes outside the target editing area, meaning this technology should not be seen as a tool for anti-aging therapy, since aging is a complex process involving the intricate interaction of many genes, epigenetic factors, environment, and metabolism.
As people age, cells begin to “forget” which genes to turn on and which to keep off. This failure does not occur in the DNA itself, but in its “settings” — the epigenome. Over time, under the influence of stress, inflammation, and accumulated damage, gene function becomes disrupted, and cells begin getting the wrong signals.
In 2016, Juan Carlos Izpisua Belmonte’s team at the Salk Institute was the first to show that epigenetic settings could be reset. Scientists used four proteins, the “Yamanaka factors,” known for their ability to revert cells to a stem-like state. To control the process, animals with accelerated aging (a progeria model) were given doxycycline, which triggered the production of these factors.
The results were impressive: in the test mice, wounds healed faster, heart and pancreas function improved, and aging of the kidneys and spleen slowed. In addition, average lifespan increased, and signs of cellular aging decreased. Importantly, the cells retained their identity and did not revert to embryonic cells, which had previously been considered the main risk of such experiments.
At the same time, David Sinclair’s team at Harvard Medical School applied the same approach to restore vision in old mice. Visual neurons began to behave like young ones: damaged axons regrew, and eyesight was restored.
These studies laid the foundation for an entire field — epigenetic reprogramming in gerontology. Today, the concept is actively developing. Sinclair’s lab continues research on systemic reprogramming in mammals, while Altos Labs and Rejuvenate Bio are creating therapeutic protocols for clinical trials. However, it is still very early to talk about complete “resetting” or reversal of aging.
Myth four: rejuvenating blood transfusions
This is one of the oldest myths about combating aging. It emerged long before the advent of biochemistry and regenerative medicine. The idea is that youth can be restored by transfusing blood from a younger donor into an older recipient.
The first scientific attempt to test this idea was made in 1864 by the French physiologist Paul Bert. He conducted parabiosis experiments — the surgical joining of the circulatory systems of two animals. His experiments laid the groundwork for studying systemic factors circulating in the blood and their effects on tissue growth and aging.
In the 1920s, the Soviet scientist and physician Alexander Bogdanov promoted his own theory of rejuvenation through blood transfusions. He transfused himself with blood from young students, claiming that he felt significantly better afterward.
Bogdanov went on to found the Moscow Institute of Blood Transfusion, where researchers studied the effects of blood on health and aging. However, his personal experiments ended tragically: in 1928, he died after a transfusion from a young student, presumably due to blood type incompatibility and hemolytic shock.
In the mid-20th century, scientists revisited the parabiosis model. By joining the circulatory systems of young and old rats, American biochemist Clive McCay tested whether the age of blood affects metabolism and tissue healing. The results were interesting but inconclusive: it was impossible to distinguish the effect of young blood flow from other factors, such as stress and immune responses.
The parabiosis idea resurfaced in the 21st century. In 2005, a team led by Irina Conboy at the University of California, Berkeley, and Tony Wyss-Coray at Harvard showed that connecting the circulatory systems of young and old mice improved heart, brain, muscle, and stem cell function in the older animals. Then, in 2014, researchers at Stanford confirmed that transfusing plasma from young mice to old mice improved the latter’s memory and learning abilities.
In 2014, Stanford researchers confirmed that transfusing plasma from young mice to old mice improved the latter’s memory and learning abilities
All of these studies sparked a surge of interest in “young blood.” Riding this wave of popularity, the medical startup Ambrosia began offering paid plasma transfusions from donors aged 16–25, claiming that the procedure could slow aging and improve memory. At one point, the FDA had to issue a warning that such procedures lacked proven effectiveness and could be dangerous, and the project eventually ceased operations.
In 2023, researchers at Harvard found that the lifespan of old mice is indeed extended after parabiosis, but the effect may be due less to the influx of “young” factors and more to the dilution of harmful molecules accumulated in old blood. For example, the protein GDF11 can stimulate tissue regeneration and improve the function of blood vessels and skeletal muscles.
Today, research is focused on identifying and isolating specific molecules in blood plasma that can be used as drugs or biomarkers of regenerative potential.
Myth five: anti-aging vaccines
Stem cells are often seen as a sort of panacea — as if a single injection could instantly trigger tissue regeneration, rewind biological age, and push back aging. Of course, they are a powerful tool in regenerative medicine, but their proven successes today are localized, and injections are appropriate only for specific diseases and tissue damage.
Historically, the myth was fueled by two major discoveries: the isolation of human embryonic stem cells (ESC) by James Thomson, and the creation of induced pluripotent stem cells (iPSC) by Kazutoshi Takahashi and Shinya Yamanaka. In the 2000s, the media wave around ESC/iPSC generated expectations of total bodily renewal, and articles about celebrities undergoing stem cell “rejuvenation” courses began appearing frequently. Without regulatory approval, numerous private clinics emerged offering stem cell injections. However, such therapy did not always end well. Patients sometimes faced severe complications from the injections, including blindness.
As a result of stem cell injections, patients sometimes faced severe complications, including blindness
Clinical trials of cell therapy for age-related diseases are being conducted in various countries. However, researchers themselves approach the widespread use of stem cells cautiously, fearing that the treatment could trigger tumor growth in patients.
Myth six: telomere length is the key to youth
Each time a cell divides, the ends of its chromosomes become slightly shorter. These DNA segments — which can be imagined as plastic tips on shoelaces that protect chromosomes from wear — are called telomeres. When telomeres reach a critically short length, the cell stops dividing and enters a state of senescence. This pattern was described in 1961 by Leonard Hayflick, who discovered that human cells divide a limited number of times (about 50–60 cycles) before aging.
Later, in the 1970s and 1980s, Elizabeth Blackburn, Carol Greider, and Jack Szostak discovered the enzyme telomerase, which can rebuild telomeres. This discovery earned them the 2009 Nobel Prize and sparked a new wave of expectations: if telomerase can extend the life of a cell, could the same effect be possible for the entire organism?
Telomeres
Experiments have indeed shown that activating telomerase increases the lifespan of cells under laboratory conditions. But in a living organism, the situation is more complex. Telomeres are part of a delicate regulatory system that balances tissue renewal with tumor suppression. Almost all malignant tumors activate telomerase in order to bypass division limits, so systemic activation of this enzyme could increase cancer risk.
In 2012, researchers María Blasco and Eduardo Telesforo at the Spanish National Cancer Center demonstrated that activating telomerase in mice via gene therapy could rejuvenate tissues and extend lifespan. In one experiment, the animals lived 40% longer, and their organs appeared younger. However, higher doses of the enzyme sharply increased tumor incidence.
Later, Blasco’s team attempted to address this problem by developing temporary telomerase activation systems (for example, using adenoviral vectors that act for a limited time). The results appeared safer but remained strictly in the realm of animal experiments.
The animals lived 40% longer, but higher doses of the enzyme sharply increased the incidence of tumors
Once it became known that telomere length decreases with age and is longer in long-lived individuals, the market responded immediately. Supplements with “telomerase activators” appeared and were actively advertised as natural rejuvenating remedies. However, clinical studies showed that the effect of such products on human telomere length is minimal or statistically insignificant. Moreover, uncontrolled stimulation of cell division in aging tissues can cause mutations or inflammation.
Scientists are gaining a deeper understanding of the root causes of aging. They have learned to determine biological age and are exploring various ways to slow the “wear and tear” of the body. This topic is so popular that every new successful experiment generates a strong media response, often distorting the original findings. In the coming decades, we should expect not immortality, but progress in early diagnosis, prevention, and personalized approaches that will extend the healthy period of life — not to 150, but certainly above levels common today.